3D printed products for use in or on people should be made of biocompatible materials that won’t trigger an allergic or toxic reaction. But what does biocompatible mean when it comes to 3D printing materials and which ones are biocompatible?
There are more biocompatible material options available for 3D printing today than ever before enabling dental and medical professionals — as well as a wide variety of manufacturers — to 3D print products designed for short- or long-term skin contact (wearables, COVID test swabs, orthotics, earbuds, personal protective equipment) or for use inside the human body (dentures, joint replacements, bone implants, vascular stents). Even medical device development firms use biocompatible materials to 3D print their product prototypes.
Let’s explore the many types and uses of biocompatible 3D printing materials by looking at two key application areas, which can work as models for countless other applications: dental work and prosthetics. Later we’ll touch on what permanent body implants, such as knee replacements and cranial implants, are 3D printed out of.
Of course, this overview of biocompatible materials is not intended to provide medical guidance of any kind, and keep in mind that regulations on biocompatibility vary from the US to the EU to Asia and India. If you’re considering using 3D printing to manufacture and bring to market a medical device, such as an orthopedic brace or medical model, check out these guides below:
Biocompatible Dental Materials
The dental industry as a whole has quickly adopted 3D printing for a long list of products. From models to temporary dentures to permanent implants, using both plastics and metals, 3D printing delivers the patient-specific products dentists need at the speed and price consumers are looking for. In fact, according to Zion Market Research, the global dental 3D printing market was worth around $3.25 billion in 2021.
Dentists typically use resin 3D printing (also called stereolithography) to produce dentures, mouth guards, surgical tools, and a host of other products that will go in the mouth either for a few moments or permanently. There’s a wide range of resins available, and when it comes to biocompatibility manufacturers boast specific classifications, evaluations, and industry standards that can seem like a secret code. Let’s take a look at what these labels and numbers really mean.
Biocompatible classes of 3D printing materials
Biocompatibility is classified in the US, according to the FDA (and in the EU according to the MDCG), as either Class I, Class II or Class III. Elsewhere in the world, there are similar systems but not the same classifications. For example, a Class II product in the US could be Class III in China because there are no international standards. In this article, we’ll be focusing on US and EU standards.
Even though you’ll see resins marketed as Class I, the materials themselves are not classified, rather a product made with the material was submitted for testing if testing was required.
Class I is a medical device with low to moderate risk that requires general controls. Almost half of all medical devices are considered Class I and 95% are exempt from any regulatory oversight. General examples of Class I medical devices, which only touch intact skin, include elastic bandages and hand-held surgical instruments. When it comes to dental 3D printing, Class I materials are typically used for try-in devices for making dental impressions.
Class II medical devices have a moderate to high risk requiring special controls. These materials may be sold in the US once the manufacturer registers and lists them with the FDA and complies with the applicable requirements. Types of Class II devices include removable skin staples and in dentistry, permanent restorations, inlays, onlays, and artificial teeth. These are products designated for use in contact with blood, body fluids, organs, tissues, and cells.
These two Classes are further broken down into subcategories (a, b, and c) based on exposure time from limited to prolonged to permanent. For example, Class IIa indicates a Class II device for limited-time use, specifically less than 24 hours. The subcategory “b” indicates prolonged use (24 hours to 30 days) and the subcategory “c” indicates permanent use (more than 30 days).
Biocompatibility isn’t just one measure but several under the International Standards Organization (ISO). Standard 10993, to be specific, and its subcategories denote types of biocompatibility. For example, ISO Standard 10993-5 is an evaluation regarding the risks of material being toxic to living cells (or cytotoxic) while 10993-10 relates to a material as an irritant or sensitizer. There are also evaluations for whether a material is capable of inducing genetic mutation, causing a rash, or inducing widespread toxicity. Your 3D printing material may meet some or all of the standards.
The Classes are based on the risk of the device, whereas ISO 10993 is based on the duration of contact and type of contact with bodily tissues and fluids, which is what matters most when choosing a device material.
Manufacturers disclose whether or not the materials, when evaluated and tested, met the requirements of international standards for biocompatibility. Often, manufacturers even disclose which laboratory conducted the testing.
The ISO also has other standards to look for. ISO 22112 relates to the material properties of artificial teeth for dental prostheses made (including 3D printed) with polymers or ceramics. For polymer-based crowns and veneering materials, there’s ISO 10477 and for denture base polymers there’s ISO 20795. Note that these standards are not unique to 3D printing and apply to materials used in molding and machining dental products, as well.
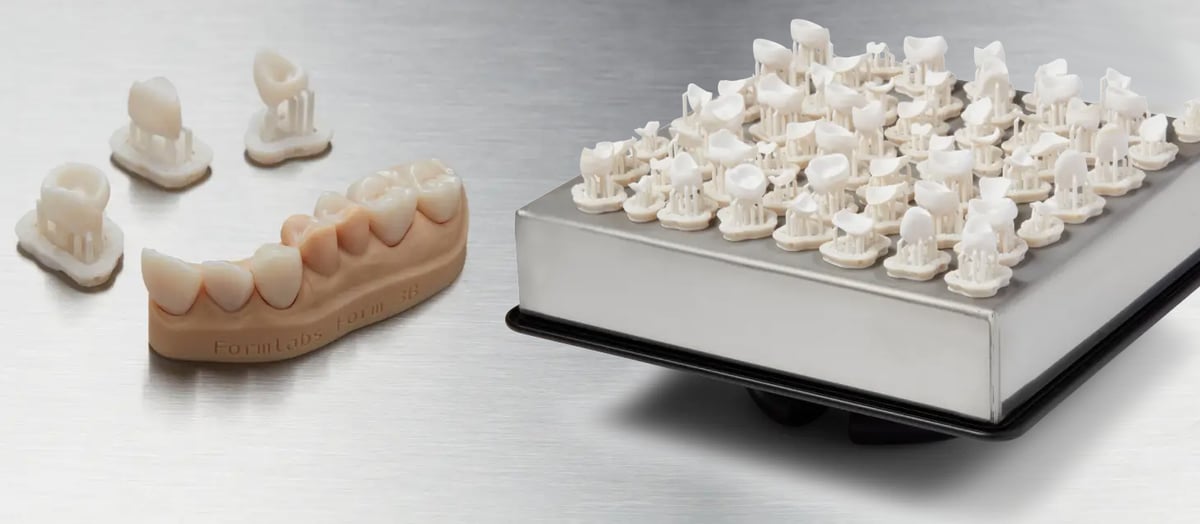
Let’s take a look at just one biocompatible 3D printing resin as an example. Denture Teeth resin from 3D printer and material maker Formlabs has an accompanying data sheet that says the material has been tested for biological evaluation of medical devices at WuXi Apptec labs in St. Paul, Minn., and is certified biocompatible per ISO 10993 to be non-mutagenic, non-cytotoxic, will not induce erythema or edema reactions, is not a sensitizer, and will not cause systemic toxicity. The post-cured properties of parts made with this material meet ISO 22112 and ISO 10477. Currently, sixteen materials from Formlabs have been publicly tested to various ISO 10993, ISO 18562, and USP Class VI endpoints with results posted at the company’s website.
Materials you 3D print with, should be able to provide the level of specificity Formlabs does either in the technical data sheets or by contacting the manufacturer.
Another designation you may see on materials is USP Class VI, which refers to a set of biocompatibility testing requirements from the U.S. Pharmacopeia (USP), a non-profit organization whose standards inform decision-making at the U.S. Food and Drug Administration (FDA). This testing is one of the most common methods to determine materials’ biocompatibility. There are six classes, with VI being the most rigorous and designed to certify that no harmful reactions or long-term bodily effects are caused by chemicals that leach out of plastic materials.
It’s not uncommon to see terms on materials that really don’t carry any weight. For example, “FDA 510(k) cleared” or “FDA 510(k)-approved” aren’t actually certifications. When the FDA clears a device through 510(k), it is not examining if the product is safe or effective for use in patients. It is just agreeing with the manufacturer’s claim that the device (or material) is similar to another already on the market. The same is true for “CE-Certified.” The letters CE on a product in the EU mean that the manufacturer attests that the product conforms with European health, safety, and environmental protection standards. It is not a quality indicator or a certification mark.
Biocompatible Resins

The list of available brands of biocompatible 3D printing resins is long and growing. These are marketed for dental applications but can also be used for other products, which may or may not have their own ISO standards to meet for mechanical properties.
Biocompatible Material for Orthotics and Prosthetics
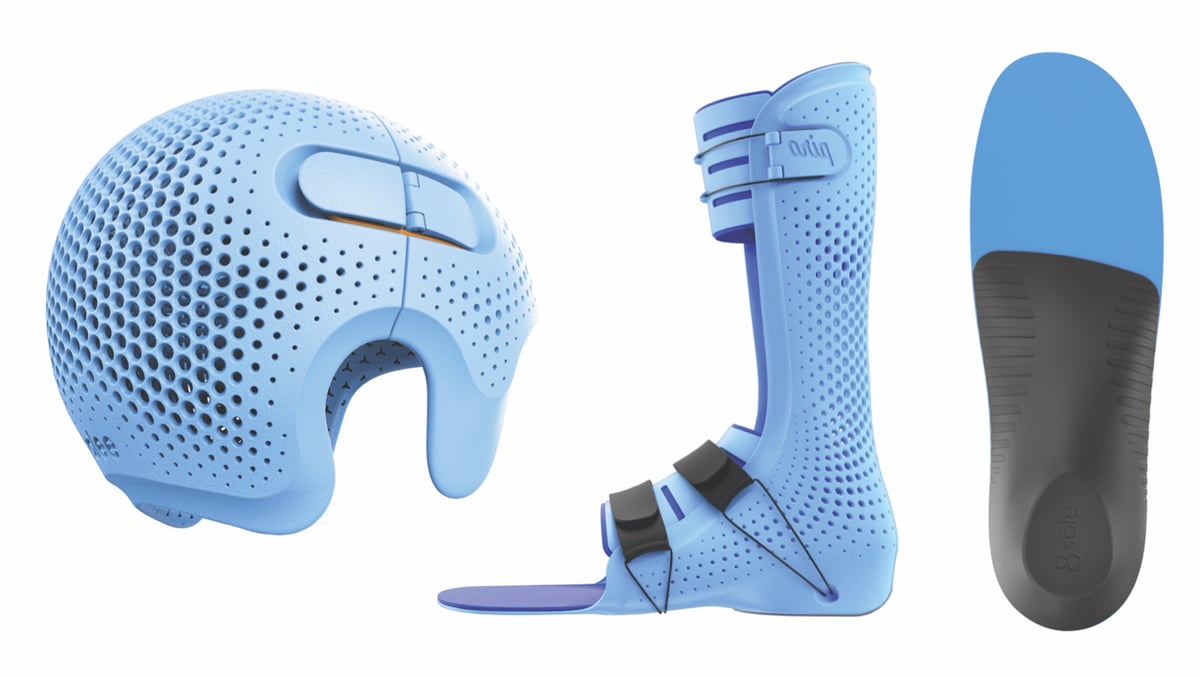
Similar to the booming market for 3D printed dental products, orthotics and braces must be 3D printed from materials certified for short- to long-term skin contact.
Whether it’s a wrist guard shaped to the patient’s unique injury or a post-op ankle splint designed around the wearer’s specific needs, producing custom-fit orthotics is a big business. A new breed of software that integrates with a wide range of available 3D scanners and 3D printers gives practitioners the ability to 3D print custom-fit products in their own offices with little to no experience in design or 3D printing.
Doctors, physical therapists, orthopedic clinics, and hospitals across the globe have new options to offer personalized products not only to lower costs and speed up delivery but to provide a better patient experience and gain a competitive edge for their business.
Companies like Belgian start-up Spentys offer software for the development of custom-made immobilization devices. Practitioners can make everything in-house or use Spentys’ modeling service and production facilities using biocompatible materials.
Orthotics are typically 3D printed using filament on FDM 3D printers due to the size of the product, but resin printers and SLS printers that use polymer powders are also options.
Biocompatible Filaments

3D printing filaments are all made with some type of polymer (even ceramic and metal filaments have some polymer). Many polymers, such as PLA, PEEK, and nylon are biocompatible by nature, but that does not mean that all PLA, PEEK, and nylon 3D printing filaments are biocompatible. There are often additives in the polymer filaments to make them perform better during 3D printing or colorants, so to make biocompatible products, you need to start with a certified biocompatible filament.
Another consideration in your polymer selection is how it stands up to sterilization and various chemical disinfectants. Some cleaning chemicals used on polymers can start to break down the material. Most manufacturers of biocompatible filaments will detail in the technical specifications whether parts 3D printed with their material, can stand up to specific chemical exposure and sterilization methods.
Below, we list just a sampling of the biocompatible filament options on the market.
Biocompatible Material for Permanent Implants

Five of the ten largest orthopedic manufacturers in the world — Stryker, Johnson & Johnson, Smith & Nephew, Zimmer, and Biomet — have turned to 3D printing for medical implants for knees, hips, spine, ankles, and more. Spinal implants are among those most commonly 3D printed, with NuVasive, SeaSpine, Tsunami Medical, and Orthofix Medical all offering 3D printed porous titanium implants for anterior lumbar interbody fusion.
Titanium is the material of choice for permanent orthopedic implants followed by cobalt-chromium alloys and stainless steel due to their excellent mechanical strength, no cytotoxicity, and good corrosion resistance. Orthopedic implants are mostly metal but sometimes ceramic (and occasionally a polymer called PEEK mentioned above).
Medical implant manufacturers typically use laser powder bed fusion 3D printing technology, either selective laser melting or electron beam melting, and sometimes directed energy deposition.
Ceramic Materials for Bone Replacement
Research and development is promising on the use of advanced ceramics for medical devices and implants with biocompatibility, bioresorbability and osteoconductivity, especially for bone replacement in regenerative medicine. Due to its properties, it is possible to manufacture patient-specific resorbable implants with defined pore structures and geometries using this material. During the healing phase, these implants will be resorbed by the body and replaced by native bone tissue, meaning that a second surgery for the removal of the implant is not necessary.
Austrian company Lithos offers a range of materials for its ceramic 3D printers that carry technical certifications according to ASTM (formerly known as American Society for Testing and Materials) standard F1185-03 (suitable for human implants) and testing to meet come ISO 10993 standards.
As 3D printing in healthcare brings new opportunities for patient-specific medical devices expect to see more biocompatible 3D printing materials hit the market.
* All3DP has done its best to research the certifications held by the products we mention and published the information provided by the manufacturers. When using a biocompatible material from any vendor, ensure its properties with that vendor directly.
License: The text of "3D Print with Biocompatible Materials" by All3DP Pro is licensed under a Creative Commons Attribution 4.0 International License.